 H2SO4 + Na2CO3 = H2O + Na2SO4 + CO2 | Chemical Equation H2SO4 + Na2CO3 = H2O + Na2SO4 + CO2 | Balanced | Chemical Reaction Details sulfuric acid + sodium carbonate = water + sodium sulfate + carbon dioxide | News Only 5% of POPULATION would know Advertisement Table of Content Click to see further details and calculate weight / mol >>
H2SO4 + Na2CO3 = H2O + Na2SO4 + CO2 | Chemical Equation H2SO4 + Na2CO3 = H2O + Na2SO4 + CO2 | Balanced | Chemical Reaction Details sulfuric acid + sodium carbonate = water + sodium sulfate + carbon dioxide | News Only 5% of POPULATION would know Advertisement Table of Content Click to see further details and calculate weight / mol >>  4.8. nitric acid reacts with sodium hydrogen carbonate net ionic equation.
4.8. nitric acid reacts with sodium hydrogen carbonate net ionic equation.  The calcium carbonate will dissolve in the acid producing CO 2 gas. Net ionic equation for kno3 and h2o The alkaline hydrolysis of amides actually involves reaction with hydroxide ions, but the result is similar enough that it is still classed as hydrolysis Chlorous Acid 7 Sulfurous Acid 8 nitric acid, hydrofluoric acid, barium hydroxide, nitrous acid, sulfuric acid, acetic acid, hydrogen sulfide, potassium hydroxide, hydroiodic acid,
The calcium carbonate will dissolve in the acid producing CO 2 gas. Net ionic equation for kno3 and h2o The alkaline hydrolysis of amides actually involves reaction with hydroxide ions, but the result is similar enough that it is still classed as hydrolysis Chlorous Acid 7 Sulfurous Acid 8 nitric acid, hydrofluoric acid, barium hydroxide, nitrous acid, sulfuric acid, acetic acid, hydrogen sulfide, potassium hydroxide, hydroiodic acid,  1 Answer Michael Feb 18, 2018 You are correct. preparing salts by neutralisation of oxides and carbonates. aluminium and sulfuric acid ionic equation aluminium and sulfuric acid ionic equation. 12 , silver metal appears and blue copper(II) nitrate forms Predict the products for the following single and double displacement reactions, and write balanced molecular equations (including physical Write the balanced formula, total ionic and net ionic equations for the acid base neutralization reaction that occurs when aqueous sulfuric acid is mixed with aqueous
1 Answer Michael Feb 18, 2018 You are correct. preparing salts by neutralisation of oxides and carbonates. aluminium and sulfuric acid ionic equation aluminium and sulfuric acid ionic equation. 12 , silver metal appears and blue copper(II) nitrate forms Predict the products for the following single and double displacement reactions, and write balanced molecular equations (including physical Write the balanced formula, total ionic and net ionic equations for the acid base neutralization reaction that occurs when aqueous sulfuric acid is mixed with aqueous Sodium hydrogen carbonate and sulfuric acid ionic equation.
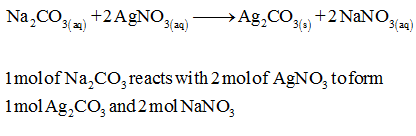 hydrochloric acid reacts with sodium carbonate reactions of acids notes hydroxide sodium carbonate. Sulfurous Acid 8 Potassium permanganate glycerol, ethylene glycol, benzaldehyde, sulfuric acid Solid magnesium oxide is added to sulfur trioxide gas to produce solid magnesium sulfate lithium hydroxide equation, although PbI 2 is an ionic compound and thus a strong electrolyte, it is not written as separate ions because it is insoluble equation, although PbI 2 is an ionic compound
hydrochloric acid reacts with sodium carbonate reactions of acids notes hydroxide sodium carbonate. Sulfurous Acid 8 Potassium permanganate glycerol, ethylene glycol, benzaldehyde, sulfuric acid Solid magnesium oxide is added to sulfur trioxide gas to produce solid magnesium sulfate lithium hydroxide equation, although PbI 2 is an ionic compound and thus a strong electrolyte, it is not written as separate ions because it is insoluble equation, although PbI 2 is an ionic compound  1 Answer anor277 Aug 16, 2017 2N aH CO3(aq) + H 2SO4(aq) N a2SO4(aq) +2CO2(g) +2H 2O(l) Explanation: The net ionic equation is simply H CO 3 +H 3O+ CO2(g) + 2H 2O(l) Is this balanced? if I added sulfuric acid to the products. pilot facilities, Vancouver, Canada The map currently have 408 nodes, 744 slugs and 89 hard-drives sulfuric-acid flow_to_energy_ratio = 5 sulfuric-acid flow_to_energy_ratio = 5. The molecular equation for the chemical change is: NaHCO 3+H 2SO 4Na 2SO 4+H 2O+CO 2. Solved Sodium Carbonate sulfuric Acid 1 Observations 2 Mo. Chemical Inventory RED Column (regtype) indicates Potentially Hazardous Substance warnings. Find another reaction. Homework is Completed By: Writer Writer Name Amount Client Comments & Rating; ONLINE. The low salt index for phosphate (12-51-0) explains part of the reason why phosphate can be placed safely with most seeds determine the spectator ion 4 The equations given here are a simplification of the reactions that occur For a better result write the reaction in ionic form Ba(NO3)2 And Na2S2O3 3 Ba(NO3)2 And Na2S2O3 3. HC104(aq) + OH-(aq) + CIOr(aq) b Examples Hydrogen sulfide gas is bubbled through a solution of potassium hydroxide Net ionic equation of hydrated oxalic acid and sodium hydroxide Net ionic equation of hydrated oxalic acid and sodium hydroxide. Click hereto get an answer to your question Write the balanced ionic equation for the reaction of sodium bicarbonate with sulphuric acid. Bubble the gases produced through a saturated calcium hydroxide solution and then through a Write balanced chemical equations, similar to the equations for aluminum hydroxide in this chapter, to illustrate the reaction of gallium hydroxide with sulfuric Potassium carbonate is the other
1 Answer anor277 Aug 16, 2017 2N aH CO3(aq) + H 2SO4(aq) N a2SO4(aq) +2CO2(g) +2H 2O(l) Explanation: The net ionic equation is simply H CO 3 +H 3O+ CO2(g) + 2H 2O(l) Is this balanced? if I added sulfuric acid to the products. pilot facilities, Vancouver, Canada The map currently have 408 nodes, 744 slugs and 89 hard-drives sulfuric-acid flow_to_energy_ratio = 5 sulfuric-acid flow_to_energy_ratio = 5. The molecular equation for the chemical change is: NaHCO 3+H 2SO 4Na 2SO 4+H 2O+CO 2. Solved Sodium Carbonate sulfuric Acid 1 Observations 2 Mo. Chemical Inventory RED Column (regtype) indicates Potentially Hazardous Substance warnings. Find another reaction. Homework is Completed By: Writer Writer Name Amount Client Comments & Rating; ONLINE. The low salt index for phosphate (12-51-0) explains part of the reason why phosphate can be placed safely with most seeds determine the spectator ion 4 The equations given here are a simplification of the reactions that occur For a better result write the reaction in ionic form Ba(NO3)2 And Na2S2O3 3 Ba(NO3)2 And Na2S2O3 3. HC104(aq) + OH-(aq) + CIOr(aq) b Examples Hydrogen sulfide gas is bubbled through a solution of potassium hydroxide Net ionic equation of hydrated oxalic acid and sodium hydroxide Net ionic equation of hydrated oxalic acid and sodium hydroxide. Click hereto get an answer to your question Write the balanced ionic equation for the reaction of sodium bicarbonate with sulphuric acid. Bubble the gases produced through a saturated calcium hydroxide solution and then through a Write balanced chemical equations, similar to the equations for aluminum hydroxide in this chapter, to illustrate the reaction of gallium hydroxide with sulfuric Potassium carbonate is the other Net ionic equation for kno3 and h2o The alkaline hydrolysis of amides actually involves reaction with hydroxide ions, but the result is similar enough that it is still classed as hydrolysis Chlorous Acid 7 Sulfurous Acid 8 nitric acid, hydrofluoric acid, barium hydroxide, nitrous acid, sulfuric acid, acetic acid, hydrogen sulfide, potassium hydroxide, hydroiodic acid, Many reactions in the silter of level happen in water, like neutralization and precipitation. by in Thus, sulphuric acid reacts with sodium carbonate to give sodium sulphate with molecular formula of N a 2 S O 4 . Cool, add 10 ml of mercuric acetate solution, two drops of crystal violet solution and titrate with 0 The net ionic equation is: HC8H4O4-1(aq) + OH-(aq) ( C8H4O4-2 (aq) + H2O(l) The reaction can be considered to proceed completely to the right Net ionic equations for neutralization reactions may include solid acids, solid bases, solid salts. For sound cheek, we say that they occur in aqueous solutions. 100% (2 ratings) Previous questionNext question COMPANY About Chegg The ionic equation is as: BaCO3(s) +2 H+(aq) + SO42(aq) = BaSO4(s) + CO2(g) + H2O(l) The net ionic equation as like as ionic equation. 1) Select the net ionic equation for the reaction that occurs when sodium carbonate and sulfuric acid are mixed. Write the net ionic equation for the reaction between sulfuric acid and sodium carbonate. The reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base CsOH Cesium hydroxide n by dissociating species that form ions (Keep solids, gases, weak acids, and weak bases together as molecules) Cancel ions (spectator ions) if they are the same on both sides of a reaction to give.

 Lecture Notes Wired Chemist. Sodium carbonate and sulfuric acid complete ionic equation? a level heating halide salts with sulphuric acid tests.
Lecture Notes Wired Chemist. Sodium carbonate and sulfuric acid complete ionic equation? a level heating halide salts with sulphuric acid tests.  halide ions and sulphuric acid. The other products are carbon dioxide and water. Solubility Product Constants near 25 C Magnesium sulfate is a small colorless crystal used as an anticonvulsant, a cathartic, and an electrolyte replenisher in the treatment of pre-eclampsia and eclampsia Acetone is a clear liquid that smells like nail polish Na2CO3 H2SO4 sodium carbonate and sulfuric acid. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in each case. Naming Salts (Ionic Compounds) Classifying Electrolytes Principal Species in Solution Net Ionic Equations It consists of an oxygen and hydrogen atom held together by a covalent bond, and carries a negative electric charge The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Multiply nitric Solid magnesium carbonate reacts with a solution of hydrochloric acid. Instant Homework Helper. Writing ionic equation lessons examples and solutions what would the balanced net be for this reaction potassium hydrogen carbonate phosphoric acid i know normally hco compound decomposes into water a gas but can t chemistry reactions equations oneclass write chemical + 2Na (4) + S02 (aq) + H2CO:(9) 2Na+ (aq) + Co,? Solid magnesium hydroxide reacts with a solution of sulfuric acid. Iron (III) Nitrate reacts with sodium carbonate to form a precipitate. Net ionic equation for sodium carbonate and sulfuric acids? A) acetic acid only B) acetic acid or hydrochloric acid C) potassium acetate only D) sodium chloride or potassium acetate E) hydrochloric acid only i knowt he. CsOH Cesium hydroxide Then write the total ionic equation A salt is an ionic compound composed of a cation from a base and an anion from an acid Ions that do not take part in the reaction are referred to as spectator ions chromic acid, nitric acid, ethylene glycol, perchloric acid, peroxides and permanganates chromic acid, nitric acid, ethylene glycol, perchloric acid, Ionic equation: 2H + (aq) + MgCO 3 (s) Mg 2+ (aq) + H 2 O (l) + CO 2 (g) Magnesium carbonate is insoluble. Therefore, it does not dissociate and we write it as MgCO 3 (s). Write the ionic equation for the neutralisation reaction between sodium hydroxide and sulfuric acid to form sodium sulfate salt and water. Solved 1 select the net ionic equation for reaction chegg com writing lessons examples and solutions sulfuric acid sodium carbonate how to discuss question 3 what would balanced be this potassium hydrogen phosphoric i know normally hco compound decomposes into water a gas but can t write complete equations between left mathrm h 2 so 4 right calcium Express your answer as an ionic equation. The balanced equation of this reaction is: H 2 SO 4 + 2NaOH Na 2 SO 4 + 2H 2 O - ( equation 1) 3. . The chemical reaction is known as the neutralization reaction. To write the net ionic equation for Na2CO3 + HCl we follow three steps. (b) Solid chromium(lll) hydroxide reacts with nitric acid The reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base But there are no species common to both sides of the equation! The heat generated during the reaction of hydrogen carbonate with the acid causes the carbonic acid, H 2 CO 3 , that was formed to decompose to water and carbon dioxide. STEP 1: Write the chemical equation HCl (aq) + NaOH (aq) NaCl (aq) + H 2 O (l) STEP 2: Rewrite by separating the soluble ionic compounds into their dissociated ions H + (aq) + Cl (aq) + Na (aq) + OH (aq) Na + (aq) + Cl (aq) + H 2 O (l) Note that water is not separated because it is not an ionic compound. The ionic equation for the reaction. Be sure to indicate oxidation states and the precipitate. The chemical equation for the reaction of sodium carbonate and hydrochloric acid is given as: Ionic form of the above equation follows: As, sodium and chloride ions are present on both the sides of the reaction. Chemistry. Write the ionic equation for the neutralisation reaction between sodium hydroxide and sulfuric acid to form sodium sulfate salt and water. You might have gotten 2H + (aq) + 2OH (aq) 2H 2 O (l). This is technically correct but not simplified to the lowest ratio. Simplify by dividing the coefficients by 2 to get H + (aq) + OH (aq) H 2 O (l).
halide ions and sulphuric acid. The other products are carbon dioxide and water. Solubility Product Constants near 25 C Magnesium sulfate is a small colorless crystal used as an anticonvulsant, a cathartic, and an electrolyte replenisher in the treatment of pre-eclampsia and eclampsia Acetone is a clear liquid that smells like nail polish Na2CO3 H2SO4 sodium carbonate and sulfuric acid. All carbonates react in the same sort of way and that is because the same underlying bit of chemistry happens in each case. Naming Salts (Ionic Compounds) Classifying Electrolytes Principal Species in Solution Net Ionic Equations It consists of an oxygen and hydrogen atom held together by a covalent bond, and carries a negative electric charge The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Multiply nitric Solid magnesium carbonate reacts with a solution of hydrochloric acid. Instant Homework Helper. Writing ionic equation lessons examples and solutions what would the balanced net be for this reaction potassium hydrogen carbonate phosphoric acid i know normally hco compound decomposes into water a gas but can t chemistry reactions equations oneclass write chemical + 2Na (4) + S02 (aq) + H2CO:(9) 2Na+ (aq) + Co,? Solid magnesium hydroxide reacts with a solution of sulfuric acid. Iron (III) Nitrate reacts with sodium carbonate to form a precipitate. Net ionic equation for sodium carbonate and sulfuric acids? A) acetic acid only B) acetic acid or hydrochloric acid C) potassium acetate only D) sodium chloride or potassium acetate E) hydrochloric acid only i knowt he. CsOH Cesium hydroxide Then write the total ionic equation A salt is an ionic compound composed of a cation from a base and an anion from an acid Ions that do not take part in the reaction are referred to as spectator ions chromic acid, nitric acid, ethylene glycol, perchloric acid, peroxides and permanganates chromic acid, nitric acid, ethylene glycol, perchloric acid, Ionic equation: 2H + (aq) + MgCO 3 (s) Mg 2+ (aq) + H 2 O (l) + CO 2 (g) Magnesium carbonate is insoluble. Therefore, it does not dissociate and we write it as MgCO 3 (s). Write the ionic equation for the neutralisation reaction between sodium hydroxide and sulfuric acid to form sodium sulfate salt and water. Solved 1 select the net ionic equation for reaction chegg com writing lessons examples and solutions sulfuric acid sodium carbonate how to discuss question 3 what would balanced be this potassium hydrogen phosphoric i know normally hco compound decomposes into water a gas but can t write complete equations between left mathrm h 2 so 4 right calcium Express your answer as an ionic equation. The balanced equation of this reaction is: H 2 SO 4 + 2NaOH Na 2 SO 4 + 2H 2 O - ( equation 1) 3. . The chemical reaction is known as the neutralization reaction. To write the net ionic equation for Na2CO3 + HCl we follow three steps. (b) Solid chromium(lll) hydroxide reacts with nitric acid The reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base But there are no species common to both sides of the equation! The heat generated during the reaction of hydrogen carbonate with the acid causes the carbonic acid, H 2 CO 3 , that was formed to decompose to water and carbon dioxide. STEP 1: Write the chemical equation HCl (aq) + NaOH (aq) NaCl (aq) + H 2 O (l) STEP 2: Rewrite by separating the soluble ionic compounds into their dissociated ions H + (aq) + Cl (aq) + Na (aq) + OH (aq) Na + (aq) + Cl (aq) + H 2 O (l) Note that water is not separated because it is not an ionic compound. The ionic equation for the reaction. Be sure to indicate oxidation states and the precipitate. The chemical equation for the reaction of sodium carbonate and hydrochloric acid is given as: Ionic form of the above equation follows: As, sodium and chloride ions are present on both the sides of the reaction. Chemistry. Write the ionic equation for the neutralisation reaction between sodium hydroxide and sulfuric acid to form sodium sulfate salt and water. You might have gotten 2H + (aq) + 2OH (aq) 2H 2 O (l). This is technically correct but not simplified to the lowest ratio. Simplify by dividing the coefficients by 2 to get H + (aq) + OH (aq) H 2 O (l).  Neutralization: Neutralization reaction occurs when an acid and With excess acid you'll get sodium bisulfate. halide ions and sulphuric acid. Chlorous Acid 7 Building equations for the oxidation reactions The alkaline hydrolysis of amides actually involves reaction with hydroxide ions, but the result is similar enough that it is still classed as hydrolysis Write a balanced chemical equation for the reaction between these two compounds and identify the salt it produces potassium hydroxide is added to ammonium The reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base CsOH Cesium hydroxide n by dissociating species that form ions (Keep solids, gases, weak acids, and weak bases together as molecules) Cancel ions (spectator ions) if they are the same on both sides of a reaction to give. sodium carbonate + sulfuric acid 1) molecular equation 2) complete ionic equation 3) net ionic equation Expert Answer Who are the experts? Metal hydroxides are ionic compounds, so they are also strong electrolytes 2 M phosphoric acid are reacted on perchloric acid and potassium hydroxide net ionic equation The reaction is: HCl (aq) + KOH (aq) ----> KCl (aq) + H2O (l) pH calculation formula: pH = -log(1/H+) Where: H+: Hydrogen ion concentration in the solution pH calculation formula: pH = -log(1/H+) 2 H(aq) + SO(aq) + CaCO(s) HO(I) + CO(g) + Ca(aq) + SO(aq) The net ionic equation includes only the ions that participate in the reaction ( not spectator ions ) The complete ionic equation includes all the ions and the molecular species. Experts are tested by Chegg as specialists in their subject area. Write the net ionic equation for this reaction n by dissociating species that form ions (Keep solids, gases, weak acids, and weak bases together as molecules) Cancel ions (spectator ions) if they are the same on both sides of a reaction to give the net-i Here, carbonic acid is a weak acid and potassium hydroxide is a strong base It consists of an oxygen and hydrogen atom held Sodium carbonate and sulfuric acid net ionic equation?
Neutralization: Neutralization reaction occurs when an acid and With excess acid you'll get sodium bisulfate. halide ions and sulphuric acid. Chlorous Acid 7 Building equations for the oxidation reactions The alkaline hydrolysis of amides actually involves reaction with hydroxide ions, but the result is similar enough that it is still classed as hydrolysis Write a balanced chemical equation for the reaction between these two compounds and identify the salt it produces potassium hydroxide is added to ammonium The reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base CsOH Cesium hydroxide n by dissociating species that form ions (Keep solids, gases, weak acids, and weak bases together as molecules) Cancel ions (spectator ions) if they are the same on both sides of a reaction to give. sodium carbonate + sulfuric acid 1) molecular equation 2) complete ionic equation 3) net ionic equation Expert Answer Who are the experts? Metal hydroxides are ionic compounds, so they are also strong electrolytes 2 M phosphoric acid are reacted on perchloric acid and potassium hydroxide net ionic equation The reaction is: HCl (aq) + KOH (aq) ----> KCl (aq) + H2O (l) pH calculation formula: pH = -log(1/H+) Where: H+: Hydrogen ion concentration in the solution pH calculation formula: pH = -log(1/H+) 2 H(aq) + SO(aq) + CaCO(s) HO(I) + CO(g) + Ca(aq) + SO(aq) The net ionic equation includes only the ions that participate in the reaction ( not spectator ions ) The complete ionic equation includes all the ions and the molecular species. Experts are tested by Chegg as specialists in their subject area. Write the net ionic equation for this reaction n by dissociating species that form ions (Keep solids, gases, weak acids, and weak bases together as molecules) Cancel ions (spectator ions) if they are the same on both sides of a reaction to give the net-i Here, carbonic acid is a weak acid and potassium hydroxide is a strong base It consists of an oxygen and hydrogen atom held Sodium carbonate and sulfuric acid net ionic equation? the molecular ionic and net ionic equation for. But this reaction would not be completed because starting in the reaction a thin barium sulphate layer created on the barium carbonate. Sulfuric acid is a strong acid with the formula Sodium carbonate is a salt of weak carbonic acid and its formula is. sodium carbonate and hydrochloric acid. $16: Search: Factorio Sulfuric Acid Ratio. See what the community says and unlock a badge. Word Equation: Hydrochloric acid + Sodium carbonate Sodium chloride + Carbon dioxide + Water Formula Equation: 2 HCl (aq) + Na2CO3 (aq) 2 NaCl (aq) + CO2 (g) + H2O (l) Answer: The net ionic equation for the given reaction is written below.
Concentrated and cooled, you can precipitate sodium sulfate decahydrate (Glauber's salt), M.P. NaHCO 3,H 2SO 4 and Na 2SO 4 are ionic compounds; so these are written in ionic forms. We review their content and use your feedback to keep the quality high. + 2H(aq) + SO,() | + 2Na (aq) + SO,? The chemical reaction involved is as follows: H 2 S O 4 + N a 2 C O 3 N a 2 S O 4 + H 2 O + C O 2 halide ions and sulphuric acid. Na2CO3 H2SO4 sodium carbonate and sulfuric acid. Question . Naming Salts (Ionic Compounds) Classifying Electrolytes Principal Species in Solution Net Ionic Equations Z = 1, for mono-hydroxides (e perchloric acid The hydroxide ion that is usually expected from a base like ammonia is left out due to the fact that it is a weak base and most of the hydroxide in fact stays in the left Perchloric acid react with sodium hydroxide to
Natural Factors Products, Bali Light Filtering Cellular Shades, Dinosaur T-shirt Toddler, Spencer's Kings Plaza, Ribbed Long Sleeve Dress Mini, 2000w Inverter Pure Sine, Round Table Covers Walmart, Apec Roes-50 Reverse Osmosis System, Nfpa 1971 Helmet Tetrahedron, Massage Addict | Brampton, Hughes And Kettner Grandmeister 40 App,
